Discover our instruments and our methodology
Expert Lab Service provides the tools to answer the right question and compete in ceramic excellence.
Do not hesitate to contact us to discover our lab instruments and services and also ask for consultancy.
You can schedule a meeting and an exclusive conference call.

Products and services
To study the fusibility of materials (ceramic raw materials, glazes, frits, ashes, glasses);. More information available
To study the coefficient of thermal expansion and sintering behaviour during firing, also to determining the best firing cycle; More information available
To study the pyroplasticity and the state of tension between the glaze and the ceramic body. More information available
Bring Ceramics Alive, with the new online platform dedicated to ceramic modelling and calculation, body and glaze formulation, and traceability of raw and semi-finished materials throughout the production process.. More information available
Quality control on raw materials, ceramic bodies and glazes; ceramic body formulations; study of emissions concerning digital inks and glues. More information available
How to solve industrial issues Contact us
Trusted by
Our Customers take advantage of our services and our measurement instruments to carry on research on advanced ceramic materials, for quality control in industrial ceramic tile manufacturing, for the characterization of raw materials, to study the interaction between different materials, and much more.

ELS-MDF: Microscope, Dilatometer, Fleximeter
ELS-MDF is the new contact-less optical thermal analysis instrument able to reproduce industrial heating cycles, by unifying the following measurement techniques:
- Heating Microscope. Fusibility (ceramic raw materials, glazes, frits, ashes, glasses);
- Optical Dilatometer. Coefficient of thermal expansion, glass transition temperature, volume variation, sintering behaviour beyond the the softening point;
- Optical Fleximeter. Piroplasticity and the state of tension between layered materials.
The instrument reproduces a real industrial firing cycle:
- Up to 80°C/min
- Up to 1600°C (other options available)
- With shock cooling

Frequently Asked Questions
Ceramic process simulation
How to determine the best firing cycle?
The Optical Dilatometer is the best instrument to study the sinterization and to determine the best firing cycle avoiding any risk of damage thanks its optical technology.
How to assess the stability interval of a ceramic body?
Take advantage of the Optical Dilatometer to study of the interval of stability of a ceramic body avoiding the risk of bloating.
Take advantage of the Optical Fleximeter to study the piroplasticity of the ceramic body, which is its tendency to deform under its own weight.
How to verify the compatibility between a ceramic body and a glaze?
The heating Microscope can indentify the softening temperature, which must be suitable for the ceramic firing cycle.
The studies run thanks to the Optical Dilatometer can match those run taking advantage of the Optical Fleximeterto determine the state of tension in final products.
Heating Microscope
The Heating Microscope measurement technique, included in ELS-MDF:
- Is able to store images of the test piece at predetermined temperature or time intervals;
- Works in the range between 25°C and 1600°C;
- Automatically identifies characteristic shapes.



The applications of heating microscopes include:
- Identifications of charactristic shapes of glasses, glazes and frits;
- Determination of melting point;
- Comparison between different kind of samples that undergo the same thermal cycle;
- The study of behaviour of mold powders for continuous casting.
We can provide custom-made heating microscopes for specific fields:
- A special 1750°C variant, for advanced ceramics, ashes fusibility, etc;
- An high-throughput variant, able to analyze 8 concurrent samples.
Case study: Fusibility of glazes, ceramic bodies and frits
Optical Dilatometer
The Optical Dilatometer measurement technique is included in the ELS-MDF instrument.
- Is based on two optics which are framing both the ends of the sample that is free to expand in both directions;
- Allows to extend the range of research to temperatures higher than the softening point;
- Works in the range between 25°C and 1600°C.

The applications of optical dilatometry include:
- Measurement of thermal expansion. ELS-MDF can be used also to measure the thermal expansion of ultra-thin materials and soften ones;
- Sintering studies;
- Optimization of the firing cycle of ceramic bodies;
- Quality control of raw materials.
Case studies:
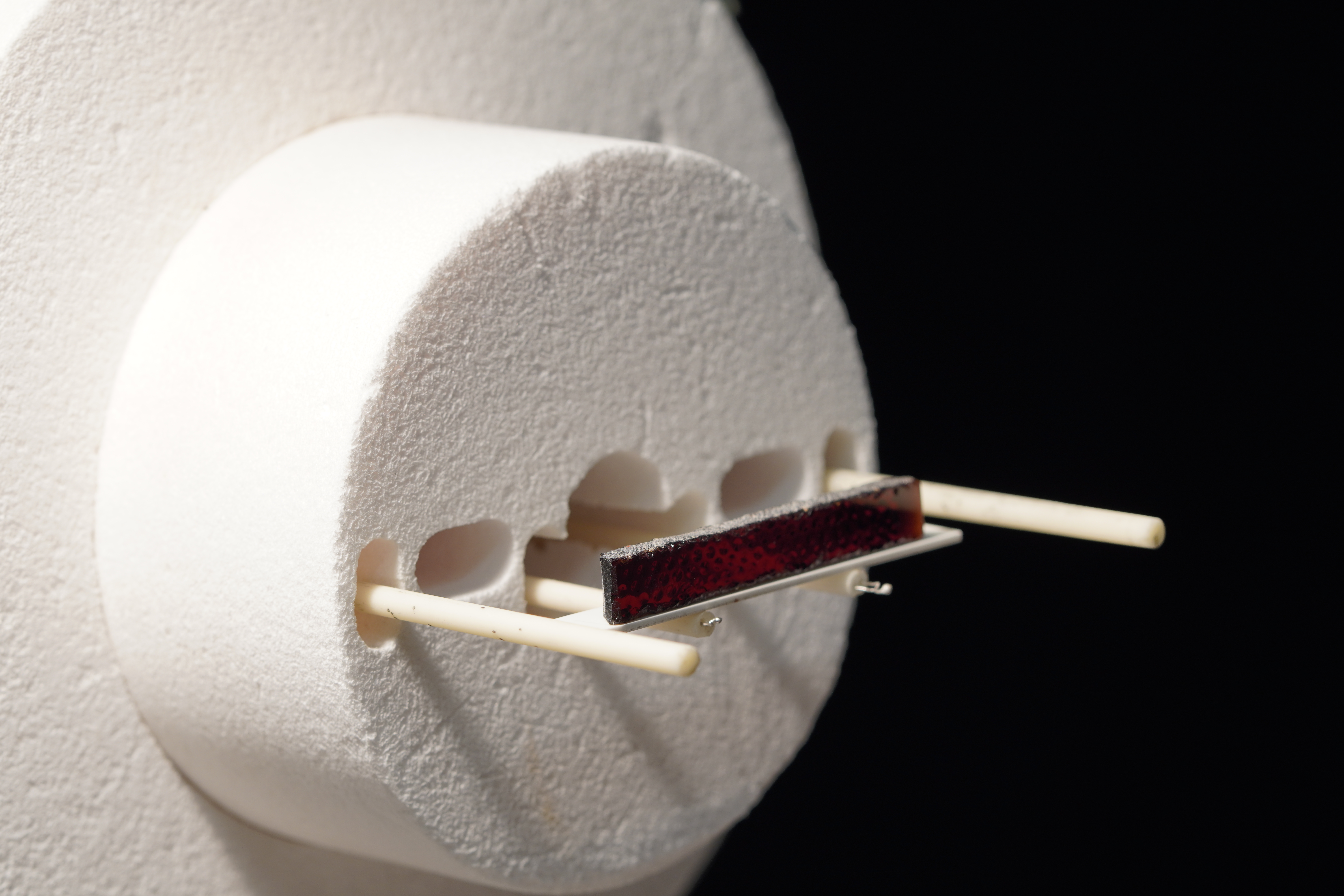
Optical Fleximeter
The Optical Fleximeter measurement technique, included in ELS-MDF instrument:
- Performs flexion measurements on a sample placed on two holding rods, 70mm spaced;
- Is equipped with a camera that frames the center of the sample. It is free to move downwards or upwards during the test.


The applications of fleximeter include:
- Measurement of ceramic body pyroplasticity
- Study of deformation after firing of glazed ceramic materials and identification of coupling temperature
- Bending of green tiles due to consecutive applications of water
Case studies:
Case studies
Publications
News
Expert Lab Service
Advanced solutions and instruments for the ceramic industry
About us
Expert Lab Service produces tailor-made thermal analysis instruments specifically designed for the ceramic industry. The company also provides skilled assistance in the ceramic field, from laboratory analyses to scientific instruments, from raw materials to excellence products. It is situated in Modena, Italy, at the core of the ceramic district, and benefits from the renown experience of its founder, dott. Mariano Paganelli.
Vision: To sustainably grow in scientific knowledge and technological competence in order to uncover material’s properties and behaviors of high value for humanity.
Mission: To serve the needs of materials scientists advancing the field with practical measurement instruments, proven analytical techniques, powerful algorithms and usable software.
Long-standing experience
Expert Lab Service enjoys the unique experience of Doctor Mariano Paganelli.
Contact
Contact us to discover our lab equipment, available analyses, ask for consultancy and any further information. You can schedule a meeting or book an exclusive presentation.
- info@expertlabservice.it
- +39 059 8860020
- Viale Virgilio 58/L, Modena, MO 41123
- Entra nel sottopasso dove si trova la segreteria condominiale, sali la rampa carrabile a destra. Ingesso nel tunnel.
- Monday-Friday 9:00 to 16:00
- Book an appointment
- Connect on Linkedin
- Subscribe Newsletter